Polymer Solutions back
back
Block copolymers in solution can self-assemble in to a variety of morphologies, with features on the nanometer length scale. This has lead to significant recent research into this assembly process and a wide range of potential applications
Stabilization of colloidal particles
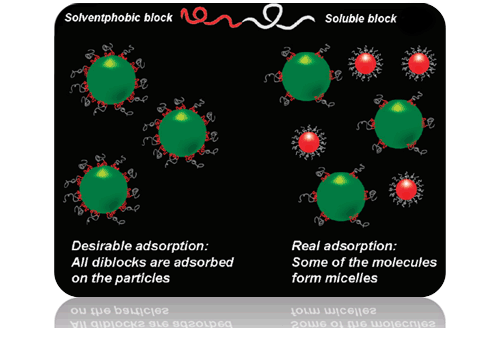
 It is known that amphiphilic (di)block copolymers are used for steric stabilization of colloidal particles: insoluble blocks are adsorbed on the surface of the particle and soluble blocks form a brush on the surface which protects the particles from aggregation. However, a competing process of micelle formation (see Figure) reduces efficiency of the copolymers as stabilizers. One needs either a long time to achieve saturation of the surface by the molecules or high amount of the polymer. To improve efficiency of polymers as stabilizers, we proposed to modify a primary structure which provides low aggregation and high adsorption abilities [1].
It is known that amphiphilic (di)block copolymers are used for steric stabilization of colloidal particles: insoluble blocks are adsorbed on the surface of the particle and soluble blocks form a brush on the surface which protects the particles from aggregation. However, a competing process of micelle formation (see Figure) reduces efficiency of the copolymers as stabilizers. One needs either a long time to achieve saturation of the surface by the molecules or high amount of the polymer. To improve efficiency of polymers as stabilizers, we proposed to modify a primary structure which provides low aggregation and high adsorption abilities [1].
Neutral micelles. Mixture of block copolymers
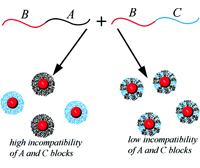
 We have developed a theory of micelle formation in solution of AB and AC diblock copolymers. Insoluble A blocks form core of the micelles, and soluble B and C blocks are corona-forming [2]. We have predicted stability of both “pure” (AB and AC) and mixed micelles, see Figure. In the mixed micelles, the B and C blocks are homogeneously mixed and can not be segregated remaining in one micelle.
We have developed a theory of micelle formation in solution of AB and AC diblock copolymers. Insoluble A blocks form core of the micelles, and soluble B and C blocks are corona-forming [2]. We have predicted stability of both “pure” (AB and AC) and mixed micelles, see Figure. In the mixed micelles, the B and C blocks are homogeneously mixed and can not be segregated remaining in one micelle.
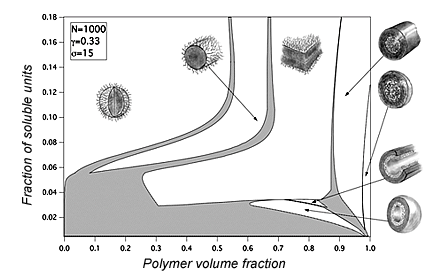
Polyelectrolyte micelles
Despite a number of theoretical studies in the field of micelle formation in solutions of diblock copolymers with a charged block, the problem of equilibrium distribution of counterions was not considered properly. We have developed a theory which takes into account the distribution of counterions depending on micellar morphology and polymer concentration [3]. This theory allowed constructing phase diagram including both dilute and concentrated regimes (see Figure). We have found that in contrast to the neutral micellar solutions, charged molecules form only spherical micelles in the dilute regime. Non-spherical micelles have higher electrostatic energy because of partial release of counterions from the corona of the micelles.