Polyelectrolytes back
back
charged polymer is macromolecule containing ionizable groups. Under appropriate conditions, such as in aqueous solutions, these groups dissociate, leaving ions on chains and counterions in solutions. if the charges on the polymers are all positive or all negative, these polymers are called polyelectrolytes. If the polymers carry both + and - groups, they are called polyampholytes.
Associating polyelectrolytes
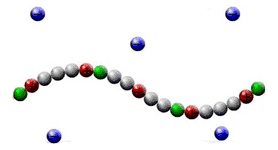 Associating polyelectrolytes are macromolecules consisting of soluble charged groups and insoluble groups (stickers) distributed along the chain. The stickers can aggregate with each other forming thermoreversible junctions. We have developed one of the first theories of geletaion [1-5] and microphase segregation [7] in solutions of associating polyelectrolytes. We have predicted new effects: stabilization of optimum size clusters in dilute solutions and so-called anomalous reversible gelation. The effect of stabilization of the clusters is due to uncompensated charge of the cluster (some of the counterions release the cluster to gain in the entropy) and related to the classical Rayleigh problem: the growth of the cluster proceeds until the electrostatic energy compensates the surface energy (the energy of the stickers) [1-5].
Associating polyelectrolytes are macromolecules consisting of soluble charged groups and insoluble groups (stickers) distributed along the chain. The stickers can aggregate with each other forming thermoreversible junctions. We have developed one of the first theories of geletaion [1-5] and microphase segregation [7] in solutions of associating polyelectrolytes. We have predicted new effects: stabilization of optimum size clusters in dilute solutions and so-called anomalous reversible gelation. The effect of stabilization of the clusters is due to uncompensated charge of the cluster (some of the counterions release the cluster to gain in the entropy) and related to the classical Rayleigh problem: the growth of the cluster proceeds until the electrostatic energy compensates the surface energy (the energy of the stickers) [1-5].
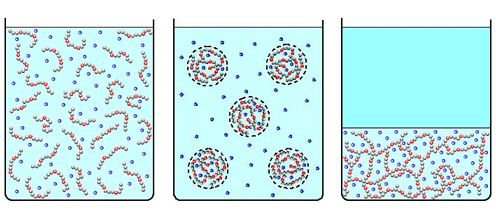
Associating polyelectrolytes can form optimum size clusters and physical gel. Some of counterions (blue dots) release the clusters increasing the entropy and violating electric neutrality of the clusters.
In contrast to neutral associating polymers, gelation in dilute solutions of the charged macromolecules can be induced by the decrease of the number of associating groups [2-4]. At the first glance, this effect is counterintuitive. However, such anomalous gelation can be explained by the presence of mobile counterions. The decrease of the number of associating groups makes the physical gel less dense. Counterions, which are trapped within the gel to provide macroscopic electric neutrality, undergo less entropic penalty than in denser gel of smaller volume. Thus, the counterions promote physical gelation at the decrease of the number of stickers on the chains. We also developed a strong segregation theory of microphase segregation in solutions of associating polyelectrolytes which predicts stability of spherical, cylindrical domains, and lamellae [7].
Liquid crystalline polyelectrolytes
The role of electrostatic interactions on liquid crystalline ordering of polymers has been discussed since famous papers by Onsager. Most of the theories are based on the mean-field approximation considering pairwise Coulomb interactions between macromolecules and leading to renormalization of the second virial coefficient. We have proposed another approach treating LC polyelectrolytes and counterions as multi-component plasma (Debye-Huckel-like approach), i.e., many-body interactions of all charged species were taken into account [6,8,16]. As a result, we have predicted orienting action of the electrostatic forces and possibility of weak nematic ordering even in dilute solutions [8]. The increase of polymer concentration results in transition from the weakly to strongly ordered nematic phase which is also stabilized by the excluded volume interactions.
Interpolyelectrolyte complexes
Mixing macroions (like charged colloidal particles) with oppositely charged polyelectrolytes, one can obtain stable complexes due to the electrostatic attraction. Under certain conditions, the total charge of the complex can have the sign opposite to the charge of the particle. In this case we can speak about overcharging (charge inversion). Efforts of many theoreticians in soft matter in the end of 1990s were mainly focused on explanation of the physical reason for overcharging of DNA-histone complexes within the models of hard particles. On the other hand, many macroions (like microgels, dendrimers, micelles, etc.), which are “penetrable” for oppositely charged polyelectrolytes, also exhibit overcharging. We have developed one of the first theories which describes the charge inversion [9] . We proposed two mechanisms of overcharging: entropic and energetic. The first one is related to the gain in entropy of mobile counterions which are released after complexation. The energetic mechanism is due to the decrease of the self-energy of excess polyions after complexation and can be demonstrated in the sketch. 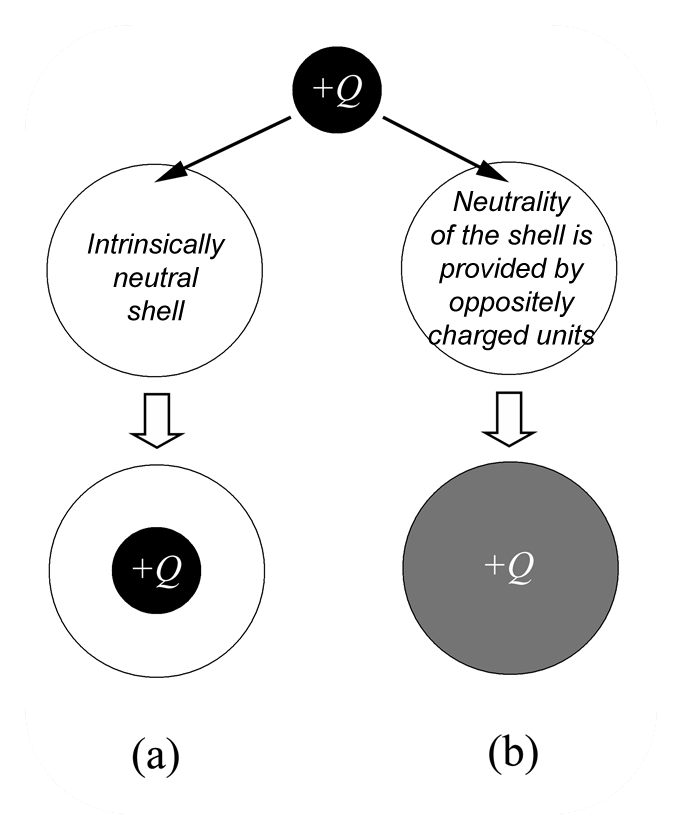 Let us imagine that a charged droplet (excess polyion) of the radius r has possibility to enter into neutral “capsules” of the radius R>r. One of the capsules is empty, and neutrality of the second capsule is provided by positive and negative charges (stoichiometric complex). The droplet in the empty capsule will have the same volume and possess the same electrostatic energy, Ea = 3Q^2/5r. On the contrary, the charge of the droplet will spread throughout the volume of the second capsule to decrease its electrostatic energy which becomes Eb = 3Q^2/5R. Thus, the energy of the bound dropled is smaller than the energy of the unbound droplet and the overcharging is energetically favorable.
Let us imagine that a charged droplet (excess polyion) of the radius r has possibility to enter into neutral “capsules” of the radius R>r. One of the capsules is empty, and neutrality of the second capsule is provided by positive and negative charges (stoichiometric complex). The droplet in the empty capsule will have the same volume and possess the same electrostatic energy, Ea = 3Q^2/5r. On the contrary, the charge of the droplet will spread throughout the volume of the second capsule to decrease its electrostatic energy which becomes Eb = 3Q^2/5R. Thus, the energy of the bound dropled is smaller than the energy of the unbound droplet and the overcharging is energetically favorable.
Also, we have studied effect of elasticity of the macroions (like microgels) on overcharging [11] and constructed phase diagrams predicting stability of overcharged complexes of different morphologies [12,13]. Formation of complexes between diblock copolymers and linear chains leading to microphase segregation was studied in ref. [14].
Polyelectrolyte micelles
Despite a number of theoretical studies in the field of micelle formation in solutions of diblock copolymers with a charged block, the problem of equilibrium distribution of counterions was not considered properly. We have developed a theory which takes into account the distribution of counterions depending on micellar morphology and polymer concentration [15].
Related articles:
J. Chem. Phys 1999, 111, 2809-2817