Ionic liquids back
back
The substances solely composed of anions and cations, that are liquid at around (and often below) room temperature are named ionic liquids
Extra solvent power
One of the first theories of ionic liquids (ILs), which takes into account both long-range electrostatic and van der Waals interactions between the ions, has been developed in our group [1-5].
We have explained extra solvent power of some of the ILs in comparison with nonionic liquids [1].
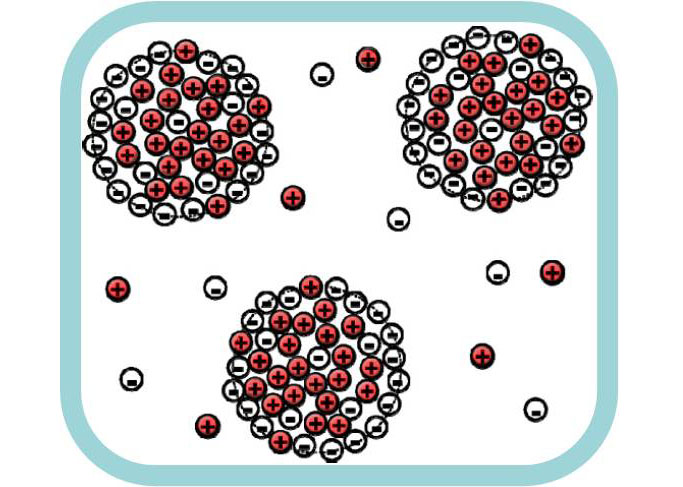
Decrease of surface tension
Other predicted effect, which found experimental confirmation, was the decrease of the surface tension at the boundary of ionic and non-ionic liquids [2]. We also demonstrated that the surface tension coefficient can become negative under certain conditions [3]. It means that the coexistence of the macroscopic phases is unstable and formation of clusters of IL in non-ionic liquid (and vice versa) is more favorable [3]. These predictions were confirmed in computer simulations.
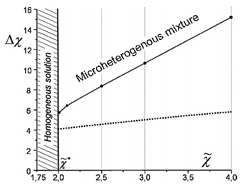
Phase diagram of the mixture of ionic and non-ionic liquids. Dotted line divides microscopic (clusters) and macroscopic phases